항생물질 다양성 개발에 합성생물학 적용 필요성

한국생명공학연원 김원곤 책임연구원
1. 개요
자연계 주로 미생물로부터 분리된 항생물질은 주로 모듈화된 생화학적 경로에 의해 생성된다. 즉, 대부분의 미생물에의해 생산되는 저분자 항생물질은 단순한 building block이 2차 대사경로를 통하여 assembly 되어 복잡한 구조로 만들어 진다. 이러한 2차 대사경로는 근본적으로 모듈화된 특성을 가지고 있어 합성생물학을 위한 훌륭한 platform이 된다.1
모듈을 디자인하여 생물학적 물질을 개량하는 합성생물학적 방법은 방선균의 2차 대사 생합성경로를 개량하는데 적용할 수 있다. 방선균등 항생물질 생산미생물은 공장의 컨베이어 벨트에서 제품이 생산하듯이 2차 대사산물을 생산하는 화학공장으로 생각할 수 있으며, 미생물의 이러한 기능은 기본 building block들에서 복잡한 구조의 화합물이 만들어 지도록 많은 효소들이 유기적으로 집적된 효소 복합체에의해서 수행된다.2
Macrolide 와 tetracycline 같은 polyketide 계 2차 대사물질은 polyketide synthase (PKS)와 라는 효소 복합체에 의해서 합성된다. 즉, acyl-coA이라는 building block이 순서적으로 또는 반복적으로 축합반응을 통하여 assembly 되어 polyketide backbone이 만들어 진다.2 이후 specific한 tailoring 효소에 의해서 sugar, alcohol, aromatic rings, methyl group, amine group의 functional group 이 부가적으로 결합한다. 이러한 functional group이 polyketide 물질의 생물활성에 주로 관여한다.3 따라서, 합성생물학적으로 PKS 와 tailoring 효소의 모듈화된 특성을 합성생물학적으로 이용하면 chemical diversity를 증가시킬 수 있다.
b-lactam, glycopeptide, lipopeptide 와 같은 nonribosomal peptide (NRPs)은 nonribosomal peptide synthase (NRPS)라는 효소에 의해 natural 아미노산 과 unnatural 아미노산을 building block으로하여 생산된다.4 NRPS 효소는 type I PKS와 비슷한 기전으로 growing peptide chain에 아미노산을 순서적으로 addition되며, tailoring 효소에의해 sugar, halogen, acyl group의 functional group 이 부가적으로 결합하기 때문에, PKS처럼 합성생물학적 방법을 적용하면 chemical diversity를 증가시킬 수 있다. 그러나, NRPS system은 building block으로 natural 아미노산 과 unnatural 아미노산을 사용하고 PKS system은 소수의 acyl CoA를 building block으로 사용하기 때문에 NRPS system이 휠씬 다양한 화학적 다양성을 줄 수 있다.
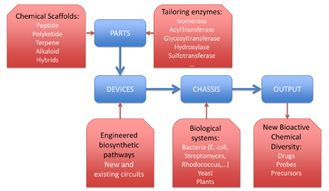
Fig. 1. 항생제개발에서 합성생물학 적용 모델
합성생물학적 용어인 part, device, chassis 를 이용하여 이상의 개념을 정리하면,5 polyketide 와 peptide 같은 scaffold를 합성하는 유전자 와 tailoring 효소는 part이고, 특성 화합물을 만들 수 있는 여러 유전자(part)가 assembly된 것이 device 이며, 이들 유전자군 (device)이 발현하고 화합물을 생산하는 적당한 host (bacteria , yeast)가 chassis이다 (Fig. 1).
2. 합성생물학적 항생제 개발
항생제 생합성 유전자는 genome 에서 일반적으로 cluster되어 있는 거대 유전자이다. Genomics, bioinformatics, analytical chemistry 기술의 발전으로 항생제와 같은 천연물 연구에 새로운 전기가 마련되고 있다.6 즉, 저비용 DNA sequencing 기술의 발전, 생합성 유전자로부터 화학구조 예측기술의 발달, 생합성 효소 와 발현 조절에 관한 지식의 증대로 합성생물학적 기술 적용이 가능하게 되었다. 이에따라 최근 10여년 동안 천연물의 생합성 경로가 많이 규명되었으며, 특히 metagenome 이나 crytic biosynthetic pathway 연구도 활발히 진행되고 있다.
합성생물학에의한 대표적인 항생제 개발은 daptomycin 연구이다. Daptomycin은 미국의 Cubist사에의해 개발된 NRPS 계 lipopeptide 항생제로서 Gram-positive bacteria에 bactericidal 항균활성을 보이고 complicated skin infection 과 내성균 감염에 사용되고 있다.7 Daptomycin은 in vitro에서 Streptococcus pneumoniae에 MIC 0.06 ug/mL의 강력한 항균활성을 보이지만, pulmonary surfactant와 결합하여 daptomycin활성이 저해되어 pneumoniae infection 에 사용할 수 없다. Baltz등은 각기 다른 아미노산의 specific 모듈을 치환함으로써 다양한 hybrid NRPS 화합물은 만들었다. Tridecapeptide backbone에서 6개의 아미노산까지 치환하여 16개의 hybrid NRPS를 만들었으며,8 이중 한 유도체가 1% surfactant 에서도 daptomycin 보다 32배 낮은 MIC를 보였으며, S. pneumoniae lung infection mouse model 에서도 치료효과를 보이는 것이 발견되었다.
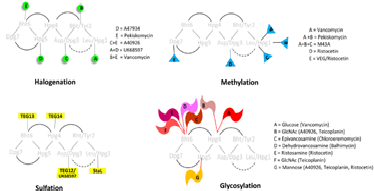
Fig. 2. Tailoring 효소에의한 glycopeptide의 다양한 modification 방법
Glycopeptide는 화학적 다양성을 증가시키기 위하여 합성생물학을 적용할 수 있는 좋은 조건을 갖추고 있다. Vancomycin, teicoplanin 과 같은 glycopeptide는 peptidoglycan 의 acyl-D-ala-D-ala 말단에 결합하여 그람 양성 세균의 생육을 저해하는 항생제로 Staphylococcus aureus 와 Entercoccus에 대하여 임상적으로 중요한 항생제이다. 이 항생제는 heptapeptide scaffold에 glycosyltransferase, halogenase, acylase, methytransferase, sulfotransferase 등의 tailoring 효소에 의해 modification 되면서 생산된다.9 Chlorination 에의한 halogenation은 peptide의 assembly 단계에서 화합물의 stability를 유지시키며, 항균활성에 필요한 구조인 intermolecular dimerization 형성에 주요한 역할을 한다. 주로 2번 또는 6번 아미노산인 tyrosine 또는 -hydroxytyrosine 에 chlorine에 결합되는데, 다른 아미노산에 chlorine이 붙이면 화학적 다양성을 증가시킬 수 있다. Glycosylation은 가장 많이 연구된 tailoring 기전으로서 glycopeptide의 solubility, 구조적 rigidity, 항균활성에 영향을 미친다. 4, 6, 7번의 아미노산 특히 4번 hydroxyphenylglycine의 OH그룹에서 glycosylation 이 잘 일어난 것으로 알려져 있다. Chloroeremomycin의 glycosylatransferase (GtfB)의 결정구조가 밝혀져서 당과 aglycon의 결합위치를 modeling을 통하여 얻을 수 있다. 또한 vancomycin glycosyltransferase (GtfE)는 sugar substrate에 대한 기질 특이성이 크지 않아 aminosugar 등 30개이상의 natural 또는 synthetic sugar를 이용한다. 또한 GtfE는 유사한 다른 aglycone을 glucosylation 시키기도 한다. 즉, Amycolatopsis orientalis에서 분리된 GtfE 유전자를 A47934 (teicoplanin-related heptapeptide) 생산균주 Streptomyces toyocaensis에 발현하여 glucosyl A47934 라는 hybrid glycopeptide 생산이 보고되었다.10 이와 같이, glycosyltransferase에 대한 많은 지식이 구축되었고, glycosylation site의 다양성과 aminosugar등 suagr 기질의 다양성이 확보되어 있기 때문에, glycosylation은 합성생물학적 기술 적용 가능성이 높은 분야이다.
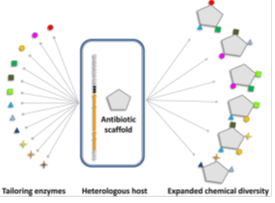
Fig. 3. 합성생물학에의한 glycopeptide 구조 다양성 확대
3.맺음말
합성생물학은 많은 천연물의 화학적 다양성을 증가시킬 수 있는 좋은 전략으로 인식되고 있다. 특히, glycopeptide는 assembly line이 잘 밝혀져 있고, 많은 tailoring 효소등이 검증되어 있기 때문에 합성생물학적 접근을 하기 좋은 scaffold이다. Amino acid를 치환하거나, glycosylation을 하여 unnatural한 hybrid glycopeptide을 성공적으로 만든 사례가 있다. 그러나, 합성생물학 기술을 routine하게 적용하기에는 여러 가지 해결해야할 도전적인 과제가 있다. 구축한 생합성 유전자군의 이종발현의 어려움, 주요 precursor의 경쟁적 대사경로 해결, tailoring 효소의 기질 특이성 및 효율, 새로운 hybrid 화합물의 efflux 문제, potential toxic 화합물의 세포내 내성 해결등이다. 그럼에도 불구하고 total synthesis를 할 수 없는 복잡한 천연물에 대하여 합성생물학은 새로운 chemistry를 제공할 수 있는 매력적인 전략이다. 더욱이, 최근에 새로운 의약물질에 대한 요구가 높고, 기존물질에 대한 새로운 활성을 찾는 연구가 커짐에 따라 합성생물학은 천연물 연구에 새로운 시대를 열 것으로 기대된다.
참고문헌
1 Khosla, C., Kapur, S. & Cane, D. E. Revisiting the modularity of modular polyketide synthases. Curr. Opin. Chem. Biol. 13, 135-143, doi:10.1016/j.cbpa.2008.12.018 (2009).
2 Lai, J. R., Koglin, A. & Walsh, C. T. Carrier protein structure and recognition in polyketide and nonribosomal peptide biosynthesis. Biochemistry 45, 14869-14879, doi:10.1021/bi061979p (2006).
3 Weymouth-Wilson, A. C. The role of carbohydrates in biologically active natural products. Nat. Prod. Rep. 14, 99-110 (1997).
4 Fischbach, M. A. & Walsh, C. T. Assembly-line enzymology for polyketide and nonribosomal Peptide antibiotics: logic, machinery, and mechanisms. Chem. Rev. 106, 3468-3496, doi:10.1021/cr0503097 (2006).
5 Koide, T., Pang, W. L. & Baliga, N. S. The role of predictive modelling in rationally re-engineering biological systems. Nat. Rev. Microbiol. 7, 297-305, doi:10.1038/nrmicro2107 (2009).
6 Lautru, S., Deeth, R. J., Bailey, L. M. & Challis, G. L. Discovery of a new peptide natural product by Streptomyces coelicolor genome mining. Nat. Chem. Biol. 1, 265-269, doi:10.1038/nchembio731 (2005).
7 Arbeit, R. D. et al. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38, 1673-1681, doi:10.1086/420818 (2004).
8 Nguyen, K. T. et al. Genetically engineered lipopeptide antibiotics related to A54145 and daptomycin with improved properties. Antimicrob. Agents Chemother. 54, 1404-1413, doi:10.1128/AAC.01307-09 (2010).
9 Kahne, D., Leimkuhler, C., Lu, W. & Walsh, C. Glycopeptide and lipoglycopeptide antibiotics. Chem. Rev. 105, 425-448, doi:10.1021/cr030103a (2005).
10 Solenberg, P. J. et al. Production of hybrid glycopeptide antibiotics in vitro and in Streptomyces toyocaensis. Chem. Biol. 4, 195-202 (1997).





